
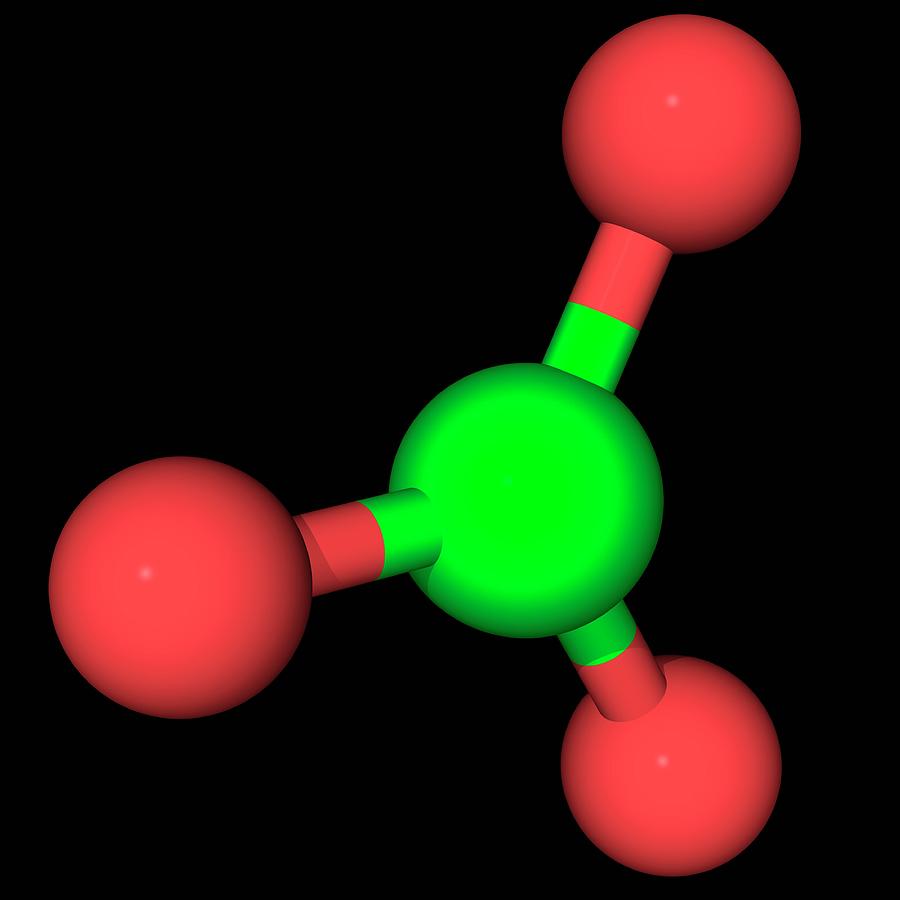
Emily Anthes, Scientific American, 1 June 2020 Always keep matches and lighter fluid out of your pets’ reach: Certain types of matches contain chlorates, which could potentially damage blood cells and result in difficulty breathing - or even kidney disease in severe cases. Draw the structural formula for the chlorate ion, ClO3-, and state the type of bonds in a chlorate ion. This is the acetate ion which is formed when acetic acid (CH3COOH) loses its proton. It is a monovalent inorganic anion and a chlorine oxoanion.

Jennifer Leman, Popular Mechanics, Farmer also suggests that people may want to avoid cleaning materials that contain peroxides, chlorates or perchlorates-all of which are oxidants that can react with a variety of common household chemicals to form toxic compounds. Chlorate is a monovalent inorganic anion obtained by deprotonation of chloric acid. Scott Gleeson, USA TODAY, 1 July 2022 Potassium chlorate breaks down into plumes of potassium chloride gas. This report is a detailed and comprehensive analysis for global Sodium Chlorate market. Structure and bonding The chlorate ion cannot be satisfactorily represented by just one Lewis structure, since all the ClO bonds are the same length (1.49 in potassium chlorate 1 ), and the chlorine atom is hypervalent. Popular Mechanics Editors, Popular Mechanics, 30 June 2022 Fireworks permitted: Cylinder fountains, cone fountains, sparklers containing no magnesium, chlorate or perchlorate snakes containing no mercury, small smoke devices. Sodium chlorate has a high oxidizing potential and functions as a powerful oxidizing agent. Generally, valence electrons can participate in the formation of chemical bonding, but core electrons cannot.Recent Examples on the Web Whoever produced these stars has mastered the barium chlorate concoction that gives fireworks the toxic-green sheen of Ghostbusters’ Slimer. S(t) of the chlorite ion clearly show a weaker hydrogen bonding at the chlorine site while the chlorate ion shows hydrogen bond life times that are much. This difference greatly influences the role of the two types of electrons in a chemical reaction. Chlorate ion or ClO3- ion comprises one Chlorine atom that is in the center forming bonds with three Oxygen atoms. This problem has been solved You'll get a detailed solution from a subject matter expert that helps you learn core concepts.
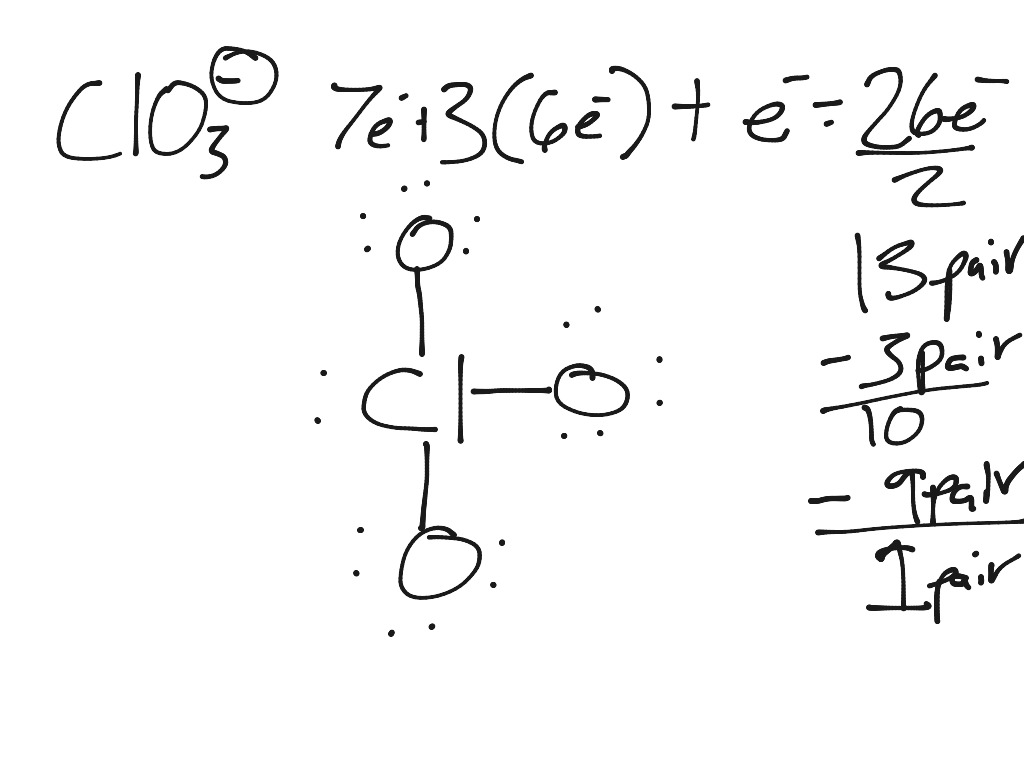
With an abundance of oxidizing elements, the Chlorate ion and its salts make for powerful oxidizing compounds. Draw the structural formula for the chlorate ion, ClO3-, and state the type of bonds in a chlorate ion. In this case, as seen in the figure, Chlorates exist in a +5 oxidation state. Chlorine can reach oxidation states of +1, +3, +5 and +7. Valence electrons are those occupying the outermost shell or highest energy level of an atom while core electrons are those occupying the innermost shell or lowest energy level. The chemical formula ClO 3 represents Chlorate ion. The electrons of an atom can divided into two categories: valence and core electrons.


 0 kommentar(er)
0 kommentar(er)
